Triple-Negative Breast Cancer (TNBC)
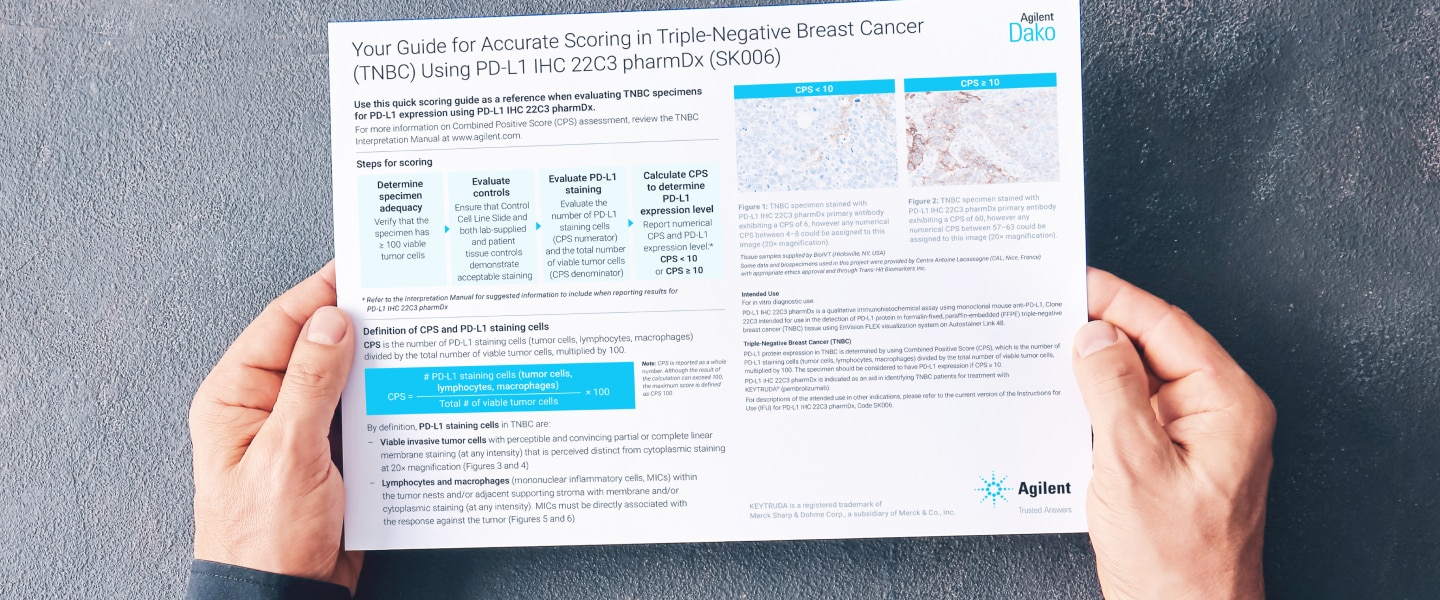
Indication-specific training resources to support high-quality diagnostic assessment of TNBC specimens stained with PD-L1 IHC 22C3 pharmDx.
PD-L1 IHC 22C3 pharmDx Interpretation Training Program for Triple-Negative Breast Cancer (TNBC)
Confidence in Evaluating Combined Positive Score: How a Systematic Approach Can Simplify PD-L1 Scoring with CPS
Scoring of Triple-Negative Breast Cancer (TNBC): Example Cases from PD-L1 IHC 22C3 pharmDx Atlas of Stain
Assessing PD-L1 Combined Positive Score (CPS) in Various Tumor Types with Distinct Morphological Characteristics
Implementation of PD-L1 Testing in the New Era of an Everchanging Immuno-oncology Landscape
The Rationale Behind the Scoring Systems for PD-L1 IHC to Determine Patients Eligible for KEYTRUDA (pembrolizumab)
D72740_01